Abstract
Background: GILT is an oral potent selective FLT3 kinase inhibitor approved for marketing for the treatment (Tx) of patients (pts) with relapsed/refractory FLT3 mutated (FLT3m) AML but efficacy in older ND FLT3m AML pts is unknown. Furthermore, FLT3m can be present as a dominant or subclone and impact of FLT3 inhibitor therapy in this setting is uncertain. Here we report the results of a Phase 2/1b sub-study of the Beat AML Master Trial to assess the efficacy of GILT monotherapy (GILTm) in ND FLT3m AML pts aged ≥60 years with high and low VAF and the subsequent response-driven addition of DEC Tx. (ClinicalTrials.gov NCT03013998)
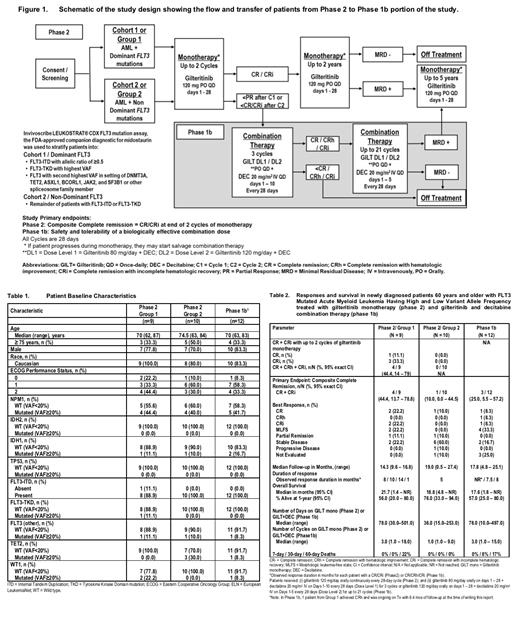
Methods: The study was an open-label multicenter (15 sites), 3-outcome, 2-stage Phase 2 design that assigned pts to either Dominant FLT3/Group 1 (GP1) or Non-Dominant FLT3/Group 2 (GP2) as shown in Figure 1. Key eligibility criteria included ND FLT3m AML pts with high and low VAF and/or ITD ratio, aged ≥60 years, and ECOG performance status 0-2. In the Phase 2 study, all pts received GILTm 120 mg/day on days 1 - 28. Pts without CR/CRi after cycle 2 were transferred to the Phase 1b study to receive GILT + DEC (Figure 1). Phase 1b study utilized a standard 3+3 design to evaluate the safety/tolerability of concurrent GILT + DEC. Pts received GILT (dose level 1 [DL1] = 80 mg/day or dose level 2 [DL2] = 120 mg/day on days 1-28) + DEC 20 mg/m 2 IV on days 1-10 or 1-5 every 28 days. Primary endpoint was CR+CRi rate (Phase 2). Response was assessed using modified 2017 ELN AML criteria. The non-dominant GP2 was stopped for futility, GP1 was stopped early to modify trial to include venetoclax.
Results: Phase 2 - Between 9/10/2018 to 2/11/2020, 19 / 20 enrolled pts (GP1: n = 9; GP2: n = 10) received GILTm and were included in analyses. Baseline pt characteristics are shown in Table 1. Median (range) time on GILTm was 3 cycles (1 - 18) in GP1 and 1 cycle (1 - 9) in GP2. Most common reasons for discontinuing Tx were Tx failure (TF; 44%) and relapse (33%) in GP1 and TF (70%) and disease progression (PD; 20%) in GP2. Overall CR+CRi was achieved in 4 pts (44%) in GP1 and 1 pt (10%) in GP2. Response duration are shown in Table 2. After median follow-up of 14.3 months (mos) and 19 mos in GP1 and GP2, respectively, 1-year OS was 56% and 76%. Most common Grade ≥3 adverse events (AEs) were febrile neutropenia and colitis (each 25%) in GP1; anemia and low platelet count in GP2 (each 30%). Overall, 7 pts had 15 serious AEs (SAEs) and all SAEs occurred in GP1 pts; most common SAE was colitis (25%) and 1 pt (13%) had a Tx-related Grade 3 SAE of tumor lysis syndrome. In GP2, 1 pt (10%) had Tx-related Grade 2 AE of differentiation syndrome. In GP1, 2 pts died within 60 days of Tx and none in GP2.
Phase1b - After up to 2 cycles of GILTm, 12 pts with no CR/CRi (GP1: n = 4; GP2: n = 8) were transferred to receive GILT + DEC (Figure 1). At the time of this report, 1 pt with CRh remained on Tx. Median total time on Tx (including GILTm) was 4 cycles and median time on GILT + DEC Tx was 3 cycles (Table 2). Most common reasons for discontinuing Tx were PD (33%) and TF (25%); and 2 pts (17%) stopped Tx due to an AE. Pts were treated with DL1 GILT + DEC (n = 3), then DL2 GILT + DEC (n = 9); only 1 pt had dose-limiting toxicity (DLT) at DL2 (Grade 3 hyperbilirubinemia and pneumonitis requiring steroid therapy), hence, DL2 GILT + DEC was considered the MTD. CR+CRi rate was 25% in 3 pts, all at DL2 (Table 2). After a median follow-up of 17.8 mos, the 1-year OS from start of GILT + DEC Tx was 57%. Most common Grade ≥3 Tx-related AEs were anemia, febrile neutropenia and low WBC count (each 22%). Overall, 6 pts had 12 SAEs; 1 pt with SAE of Grade 4 sepsis died. Three GILT-related SAEs occurred in 1 pt - Grade 3 hyperbilirubinemia, and pneumonitis and Grade 1 transaminases increased. One pt died within 30 days and a second within 60 days of Tx. No difference was observed in GILT pharmacokinetics (PK) with or without DEC, however steady state Ctrough values were 1.4 to 2.3-fold greater than in relapsed/refractory AML pts (Admiral trial).
Conclusions: In ND pts ≥60 years old with dominant FLT3 AML, GILTm induced a high 44% CR+CRi rate and long median OS (21.7 mos). Pts with non-dominant FLT3 had low 10% CR+CRi rate. GILTm was generally safe and was associated with differentiation syndrome in 1 pt. Concurrent GILT + DEC was acceptably tolerated, only 1 pt had a DLT, and the MTD was 120 mg/day GILT + DEC. A subset of pts with no CR/CRi during GILTm achieved remission with addition of DEC. Based on these results, a triple combination Tx study with venetoclax is currently enrolling.
Traer: Genentech: Membership on an entity's Board of Directors or advisory committees; Schrodinger: Research Funding; Incyte: Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier/Agios: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Membership on an entity's Board of Directors or advisory committees. Mims: Glycomemetics: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Aptevo: Research Funding; Leukemia and Lymphoma Society's Beat AML clinical study: Consultancy, Research Funding; Genentech: Consultancy; Xencor: Research Funding; Kartos Pharmaceuticals: Research Funding; Abbvie: Consultancy; BMS: Consultancy; Kura Oncology: Consultancy; Syndax Pharmaceuticals: Consultancy; BMS: Consultancy; Jazz Pharmaceuticals: Consultancy; Aptevo: Research Funding. Stein: Agios Pharmaceuticals, Inc: Consultancy; Novartis: Consultancy; Astellas: Consultancy; Syndax Pharmaceuticals: Consultancy; Daiichi Sankyo: Consultancy; Syros Pharmaceuticals, Inc.: Consultancy; PinotBio: Consultancy; Celgene: Consultancy; Bristol Myers Squibb: Consultancy; Jazz Pharmaceuticals: Consultancy; Foghorn Therapeutics: Consultancy; Blueprint Medicines: Consultancy; Gilead Sciences, Inc.: Consultancy; Abbvie: Consultancy; Janssen Pharmaceuticals: Consultancy; Genentech: Consultancy. Stock: Pfizer: Consultancy, Honoraria, Research Funding; amgen: Honoraria; agios: Honoraria; jazz: Honoraria; kura: Honoraria; kite: Honoraria; morphosys: Honoraria; servier: Honoraria; syndax: Consultancy, Honoraria; Pluristeem: Consultancy, Honoraria. Kovacsovics: AbbVie: Research Funding; Jazz Pharmaceutials: Honoraria; Janssen Pharmaceuticals: Research Funding; Amgen Inc.: Research Funding; Stemline: Honoraria; Novartis: Research Funding. Blum: Xencor: Research Funding; Abbvie: Honoraria; Nkarta: Research Funding; Celyad Oncology: Research Funding; AmerisourceBergen: Honoraria; Forma Therapeutics: Research Funding; Leukemia and Lymphoma Society: Research Funding; Syndax: Honoraria. Arellano: Syndax Pharmaceuticals, Inc: Consultancy; KITE Pharma, Inc: Consultancy. Schiller: Actinium Pharmaceuticals, Inc: Research Funding; Mateon: Research Funding; Tolero: Research Funding; Geron: Research Funding; Regimmune: Research Funding; Kite/Gilead: Honoraria, Research Funding, Speakers Bureau; Celator: Research Funding; Sangamo: Research Funding; Stemline Therapeutics, Inc.: Honoraria, Research Funding, Speakers Bureau; Takeda: Research Funding; PrECOG: Research Funding; Pfizer: Current equity holder in publicly-traded company, Research Funding; Karyopharm: Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gamida Cell Ltd.: Research Funding; FujiFilm: Research Funding; Samus: Research Funding; Trovagene: Research Funding; Daiichi-Sankyo: Research Funding; Constellation Pharmaceuticals: Research Funding; BMS/Celgene: Consultancy, Current equity holder in publicly-traded company, Research Funding, Speakers Bureau; Abbvie: Research Funding; Actuate: Research Funding; Arog: Research Funding; Delta-Fly: Research Funding; Amgen: Consultancy, Current equity holder in publicly-traded company, Honoraria, Research Funding, Speakers Bureau; Agios: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; Jazz: Consultancy, Honoraria, Research Funding, Speakers Bureau; Elevate: Research Funding; Ono-UK: Consultancy, Research Funding; Onconova: Research Funding; Deciphera: Research Funding; Astellas: Honoraria, Research Funding, Speakers Bureau; Forma: Research Funding; Genentech-Roche: Research Funding; Bio: Research Funding; Sanofi: Honoraria, Research Funding, Speakers Bureau; Pharma: Consultancy; Johnson & Johnson: Current equity holder in publicly-traded company; Biomed Valley Discoveries: Research Funding; Eli Lilly: Research Funding; ASH foundation: Other: Chair-unpaid; Sellas: Research Funding; Ono: Consultancy; Incyte: Consultancy; Ariad: Research Funding; AstraZeneca: Consultancy; Kaiser Permanente: Consultancy; Cyclacel: Research Funding; MedImmune: Research Funding; Ambit: Research Funding; Leukemia & Lymphoma Society: Research Funding; Bluebird Bio: Research Funding; Boehringer-Ingleheim: Research Funding; Cellerant: Research Funding; CTI Biopharma: Research Funding; Janssen: Research Funding; Kura Oncology: Research Funding; Pharmacyclics: Honoraria, Speakers Bureau; Millennium: Research Funding; National Marrow Donor Program: Research Funding; NIH: Research Funding; Onyx: Research Funding; Pharmamar: Research Funding; UC Davis: Research Funding; UCSD: Research Funding; Evidera: Consultancy; NCI: Consultancy; Novartis: Speakers Bureau. Olin: Astellas: Honoraria, Research Funding; Daiichi Sankyo: Research Funding; Genentech: Research Funding; Pfizer: Research Funding; Cellectis: Research Funding; Amgen: Honoraria; Abbvie: Honoraria; Actinium: Honoraria. Foran: taiho: Honoraria; syros: Honoraria; kura: Research Funding; boehringer ingelheim: Research Funding; sanofi aventis: Honoraria; trillium: Research Funding; aptose: Research Funding; abbvie: Research Funding; pfizer: Honoraria; gamida: Honoraria; actinium: Research Funding; takeda: Research Funding; certara: Honoraria; OncLive: Honoraria; bms: Honoraria; revolution medicine: Honoraria; servier: Honoraria; novartis: Honoraria; h3bioscience: Research Funding; aprea: Research Funding; sellas: Research Funding; stemline: Research Funding. Litzow: AbbVie: Research Funding; Jazz: Other: Advisory Board; Pluristem: Research Funding; Amgen: Research Funding; Omeros: Other: Advisory Board; Actinium: Research Funding; Astellas: Research Funding; Biosight: Other: Data monitoring committee. Lin: AbbVie, Aptevo Therapeutics, Astellas Pharma, Bio-Path Holdings, Celgene, Celyad, Genentech-Roche, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Novartis, Ono Pharmaceutical, Pfizer, Prescient Therapeutics, Seattle Genetics, Tolero, Trovagene: Research Funding. Patel: BMS-Celgene, Agios: Membership on an entity's Board of Directors or advisory committees; Peerview: Honoraria; Aptevo Therapeutics: Research Funding. Foster: Bellicum Pharmaceuticals: Research Funding; Macrogenics: Research Funding; Rafael Pharmaceuticals: Research Funding; Macrogenics: Consultancy; Daiichi Sankyo: Consultancy; Agios: Consultancy. Cogle: Aptevo therapeutics: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees. Vergilio: Foundation Medicine: Current Employment. Gana: The Leukemia & Lymphoma Society: Consultancy; Bausch: Current holder of individual stocks in a privately-held company. Druker: VB Therapeutics: Membership on an entity's Board of Directors or advisory committees; The RUNX1 Research Program: Membership on an entity's Board of Directors or advisory committees; GRAIL: Current equity holder in publicly-traded company; Third Coast Therapeutics: Membership on an entity's Board of Directors or advisory committees; EnLiven: Consultancy, Research Funding; Iterion Therapeutics: Membership on an entity's Board of Directors or advisory committees; Recludix Pharma, Inc.: Consultancy; Pfizer: Research Funding; Merck & Co: Patents & Royalties; Cepheid: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Nemucore Medical Innovations, Inc.: Consultancy; Bristol-Myers Squibb: Research Funding; Blueprint Medicines: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Aptose Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Amgen: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; ALLCRON: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aileron: Membership on an entity's Board of Directors or advisory committees; Vincerx Pharma: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Vivid Biosciences: Membership on an entity's Board of Directors or advisory committees. Byrd: Novartis, Trillium, Astellas, AstraZeneca, Pharmacyclics, Syndax: Consultancy, Honoraria; Vincerx Pharmaceuticals: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Newave: Membership on an entity's Board of Directors or advisory committees. Levine: Auron: Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy; Ajax: Membership on an entity's Board of Directors or advisory committees; C4 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Isoplexis: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy; Celgene: Research Funding; Roche: Honoraria, Research Funding; Zentalis: Membership on an entity's Board of Directors or advisory committees; Prelude: Membership on an entity's Board of Directors or advisory committees; Mission Bio: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Janssen: Consultancy; Gilead: Honoraria; Morphosys: Consultancy; Imago: Membership on an entity's Board of Directors or advisory committees; QIAGEN: Membership on an entity's Board of Directors or advisory committees; Lilly: Honoraria. Borate: Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicine: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz Pharma: Research Funding; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rampal: Membership on an entity's Board of Directors or advisory committees; Galecto, Inc.: Consultancy; Promedior: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal